First Model Of The Atom
Theres the Bohr model, which is most common, and the Lewis Dot.
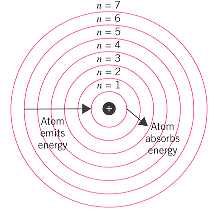
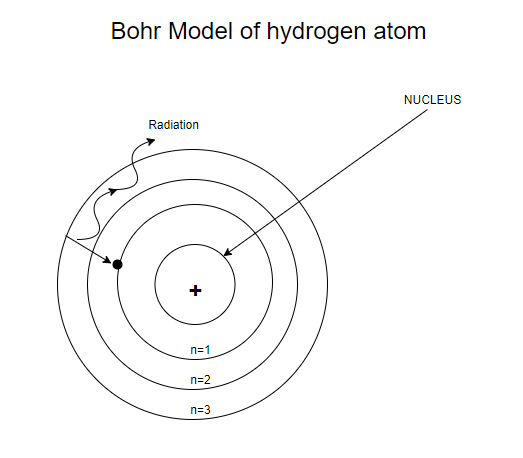
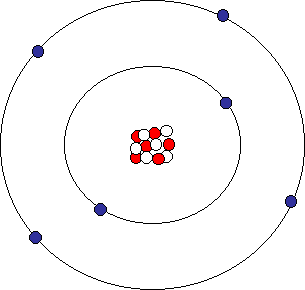
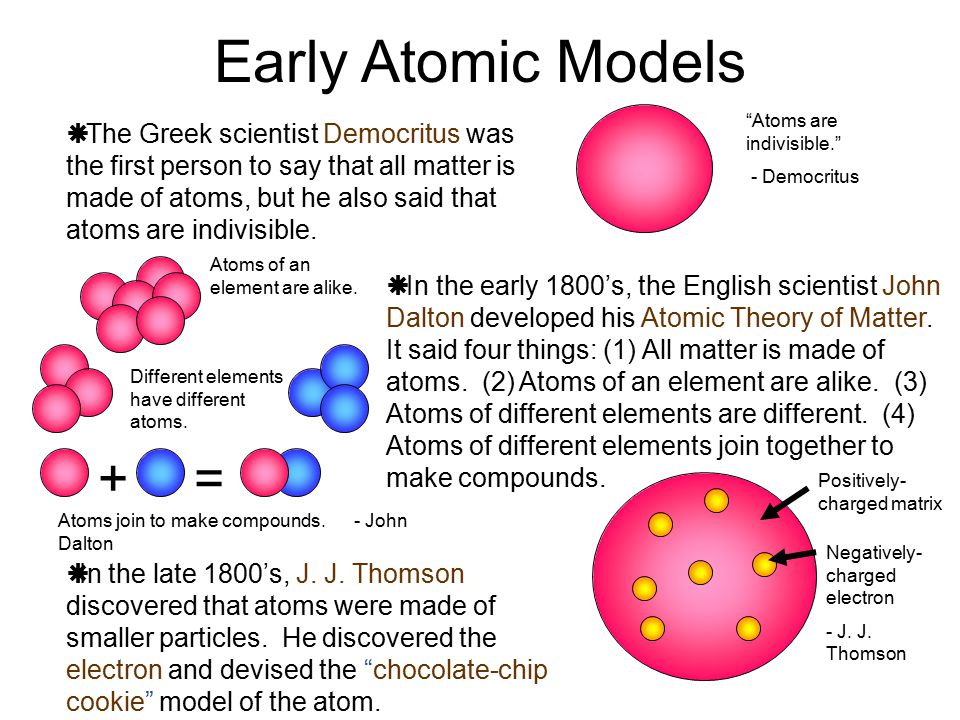
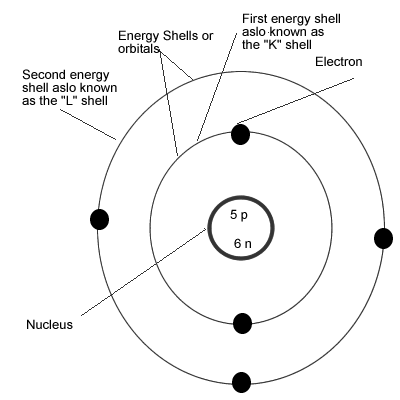
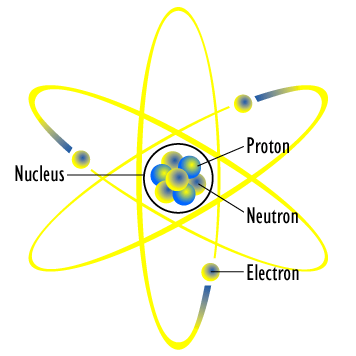
First model of the atom. In 1913, Bohr’s atomic model showed electrons orbiting an atom’s nucleus in defined energy “shells”. He was the first one to discover that all matter is made up of invisible particles called atoms. The nucleus in the atom’s Bohr model holds most of the atom’s mass in its protons and neutrons.
Give only the probability of finding an. What was thought of as a single particle about 1 × 10‾¹º m across is now known to be a. The model of the atom has changed as scientists have gathered new evidence.
He proposed that an atom is shaped like a sphere with a radius of approximately 10-10 m, where the positive charge is uniformly distributed. Bohr Model The Bohr model was devised by Neils Bohr, a physicist from Denmark who received the Nobel prize for his work on the atom. The Bohr model of the atom was the first complete physical model of the atom.
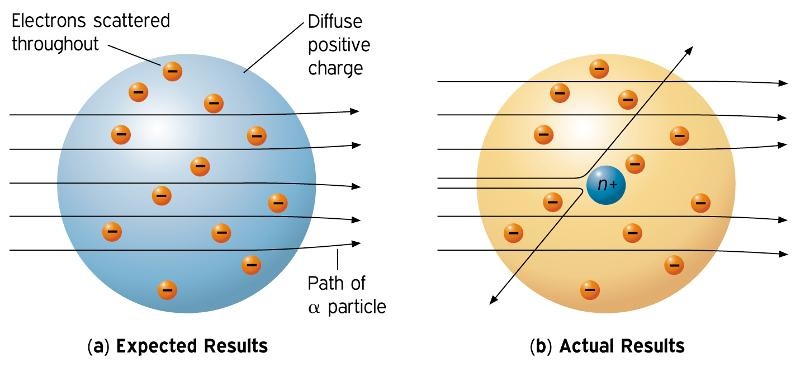
This theory was then disproved by Ernest Rutherford and the gold foil experiment in 1911, where Rutherford shot alpha particles at gold foil, and noticed that some went through and some bounced back. It described the overall structure of the atom, how atoms bond to each other, and predicted the spectral lines of hydrogen. Early Theories of Atomic Structure.
Proof of Neutron 1932. The symbol for proton number is the capital letter Z. In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.
Bohr was the first to discover that electrons move around the nucleus in different orbits and that an element’s properties are determined by the number of electrons in the outer orbit. Thomson announced his discovery of the electron and the fact that atoms must have some structure. Why did Thomson's results from experimenting with cathode rays cause a big change in scientific thought about atoms?.
Atom, smallest unit into which matter can be divided without the release of electrically charged particles. The first model of an atom was given by. Atomic theory is the scientific theory that matter is composed of particles called atoms.
Bohr’s model was first introduced in 1913. Atomic theory traces its origins to an ancient philosophical tradition known as atomism. Saturnian model of the atom.
Jerome is learning how the model of the atom has changed over time as new evidence was gathered. The first model of the atom was developed through thinking about the smallest particles of matter without experimenting. Another name for his model is the billiard ball model.
The first person to develop the first model of the atom in 1803 was John Dalton. This video is about the different ways that scientists have pictured the atoms over the years. The equation can be solved exactly for an atom containing only a single electron , and very close approximations can be found for atoms containing two or three electrons (helium and lithium).
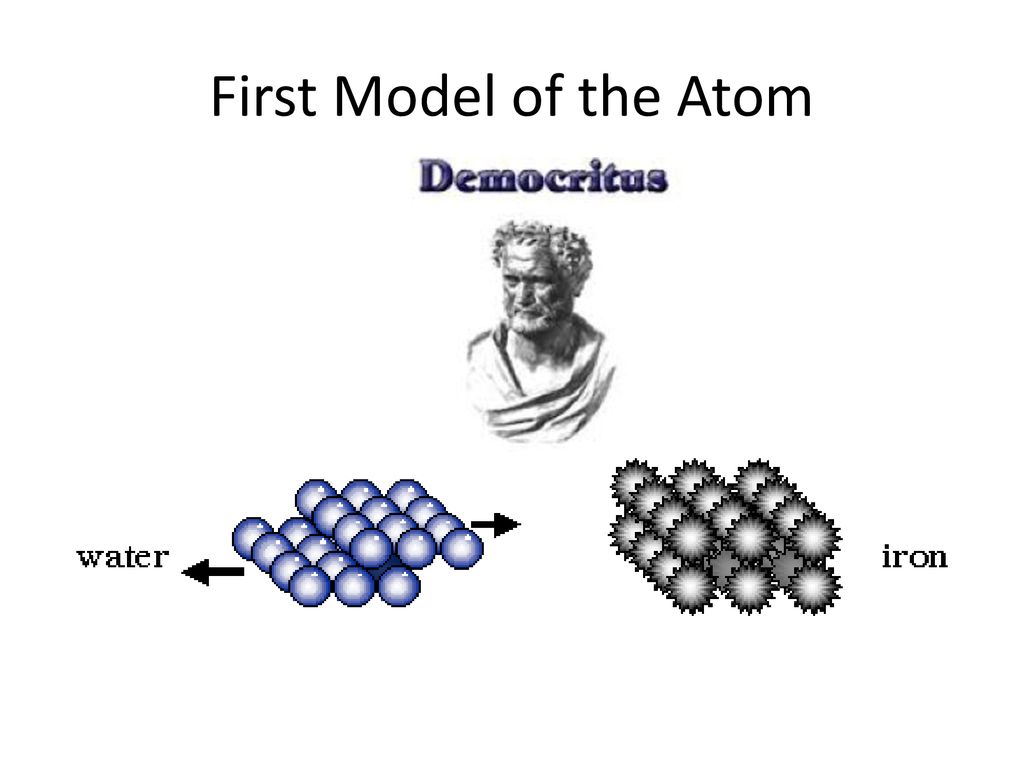
He defined atoms as tiny indivisible spherical objects that cannot be divided any further. Ancient Greek philosophers called these hypothetical ultimate particles of matter atomos, a word which meant "uncut". The first was the law of conservation of mass, formulated by Antoine Lavoisier in 17,.
Atomic models edit | edit source. The first model of atom in 18 was proposed by (a) Ernst Rutherford (b) Albert Einstein (c) J.J. The most striking property of the atom was its permanence.
By 1900 physicists had begun to consider new models for the structure of the atom. It also is the smallest unit of matter that has the characteristic properties of a chemical element. Based on the above postulates, Dalton was able to come up with one of the first models for the atom.
Despite all this, Bohr’s is probably still the model of the atom you’re most familiar with, since it’s often the one first introduced during high school or secondary school chemistry courses. Thomson (d) Niels Bohr. John Dalton created the very first atomic theory.
The model showed that the electrons orbit the nucleus just like the planets orbit the sun. Michael Fowler, University of Virginia. Models of the Atom Timeline – YouTube:.
A model of the atom that derives from the Schrödinger wave equation and deals with probabilities. Danish physicist Niels Bohr developed a modern model of the structure of atoms by integrating atomic findings of Rutherford and Max Planck’s quantum theory. The first attempt to construct a physical model of an atom was made by William Thomson (later elevated to Lord Kelvin) in 1867.
Rutherford model, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford. He was unaware at the time (as was everyone, for that matter) of the concept of the nucleus. Atoms can’t be subdivided, created or destroyed.
Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. The atomic mass of an atom is the sum of its protons and neutrons or Z + N. Democritus was the first scientist to create a model of the atom.
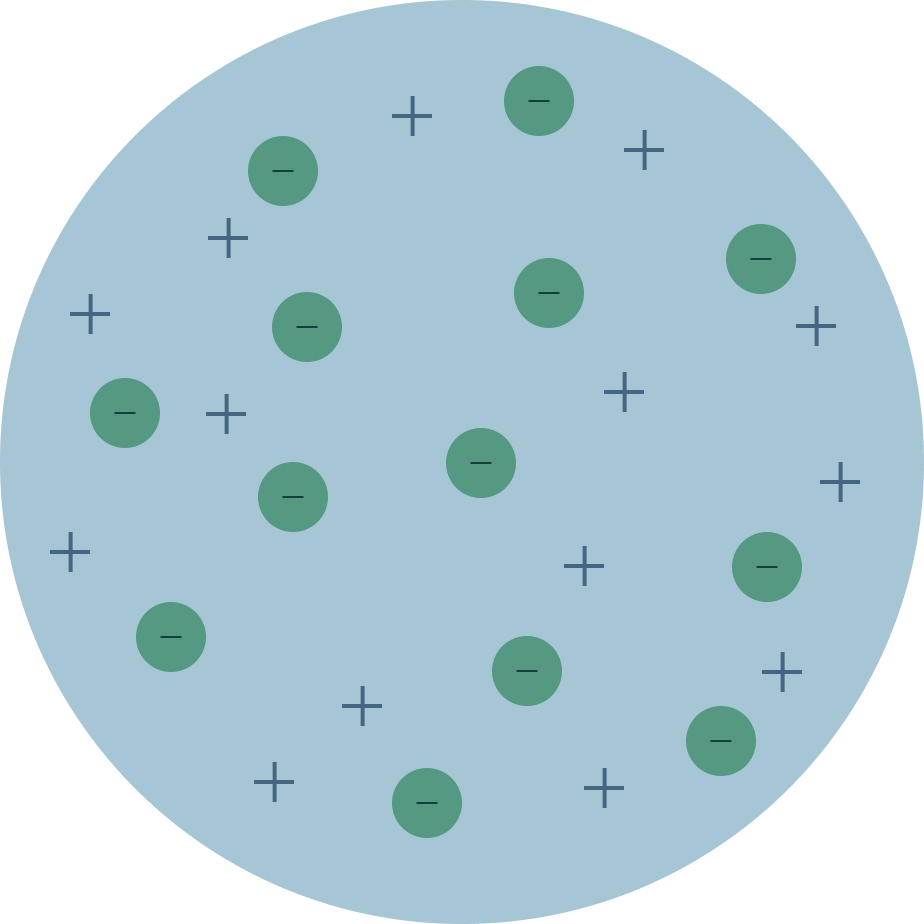
In J.J.'s model, the atom is composed of electrons surrounded by an area of positive charges to balance out the negative charges. Quantum mechanical model (1926-present) Atomic model:. The number of neutrons in an atom is indicated by the letter N.
The location of the electrons in the quantum mechanical model of the atom. He described a planetary model in which electrons orbited a small, positive-charged nucleus. Democritus' idea and use of the word "Atom" was the first step to building the foundation of chemistry with the atom thousands of years later!.
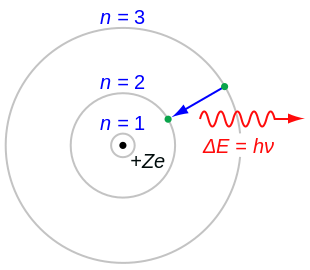
Bohr described atoms as having a positive nucleus with electrons that have different energies at different distances from the nucleus. The historical development of the different models of the atom’s structure is summarized in Figure 1.5.11 Rutherford’s model of the atom is essentially the same as the modern one, except that we now know that electrons are not uniformly distributed throughout an atom’s volume. Bohr developed the Bohr model of the atom, in which he proposed.
Ernest Rutherford, one of Thomson's students, disproved the plum pudding model in 1909. In this model, it was assumed that protons and electrons were distributed throughout the atom almost randomly. He created the name "atom" from the Greek word "atomos", which means uncuttable.
He proposed that matter could NOT be divided into smaller pieces forever. Bohr proposed, as did Rutherford, that the atom had a small, positive nucleus where most of its mass resided. This model of the atom depicts a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus much like the.
Bohr was also a philosopher and a promoter of scientific research. Image or drawing of model. This is incorrect however, considering that it is impossible to know exactly how they are actually moving.
First models of the atom Teaching Guidance for 14-16 As students start experiments on ionisation, they will have a fairly basic model of atoms and molecules, as portrayed by the simple kinetic theory. They will know that solids, liquids and gases are made up of atoms and molecules. Niels Bohr created the first successful model of an atom.
On a separate sheet of paper make a timeline of the history and development of the atom. The video also covers the work of Dalton, Thompson, Rutherford, Niels Bohr, and Schrodinger. Which scientist first made a model of the atom?.
The first model of the atom was developed by JJ Thomson in 1904, who thought that atoms were composed purely of negatively charged electrons. Thomson proposed the plum pudding model of the atom. Did Bohr’s model have neutrons?.
The Bohr model and all of its successors describe the properties of. This model has some good ideas in it, but overall it has. Scientist previously thought an atom looked like plum pudding.
The first model of the atom was developed through?. The three-dimensional region of space that indicates where there is a high probability of finding an electron. He discovered the Plum Pudding Model of the atom.
Bohr's model was not perfect and was soon superseded by the more accurate Schrödinger model, but it was sufficient to evaporate any remaining. In Geiger-Marsden scattering experiment, the trajectory traced by an a-particle depends on (a) number of collision (b) number of scattered a-particles (c) impact parameter (d) none of these. Developed the first model of the atom that showed the structure of the INSIDE of an atom.
After his retirement from Tokyo University, Nagaoka was appointed a head scientist at RIKEN, and also served as the first president of Osaka University, from 1931 to 1934. See answer sophiame48 sophiame48 Democritus was a Greek philosopher (470-380 B.C.) who is the father of a modern atomic thought. While an atom can gain or lose neutrons and electrons, its identity is tied to the number of protons.
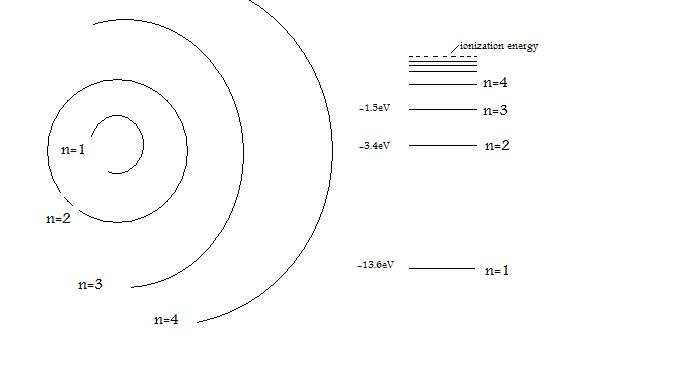
Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. John Dalton Matter is made of small indivisible atoms. It was difficult to imagine any small solid entity that could not be broken, given the right force, temperature or chemical reaction.
It starts with Democritus and Leucippus, the first philosophers to discuss atoms. The electrons are embedded in this sphere so as to give the most stable electrostatic arrangement. Thompson’s model, rutherford model, bohr model, electron cloud model 2.
Dalton’s model of the atom, based on the five points of his atomic theory. This model was known as the 'plum pudding' model. The Bohr model of the atom was a major leap forward in our understanding of atomic structure and quantum mechanics, but it was not the final answer.
In some ways it is a more sophisticated enhancement of the Rutherford model. It’s quite handy for explaining chemical bonding and the reactivity of some groups of elements at a simple level. Year and short description 3.
Models of the Atom. According to this idea, if one were to take a lump of matter and cut it into ever smaller pieces, one would eventually reach a point where the pieces could not be further cut into anything smaller. Models of the atom - AQA The idea of the atom as the building block of matter has developed over time.
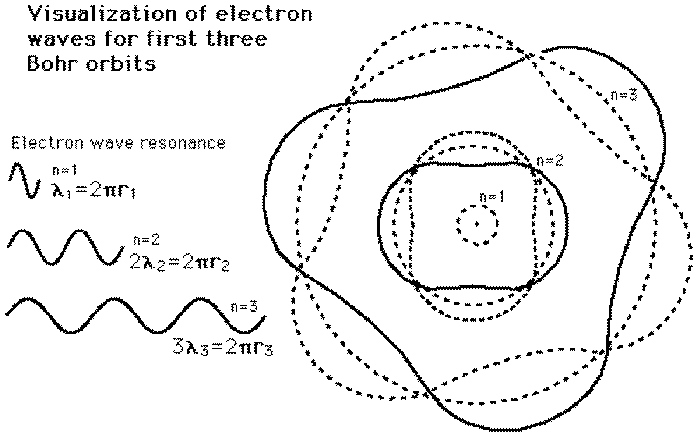
The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called electrons, circulate at some distance. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. The first model of the atom was developed through thinking about the smallest particles of matter without experimenting.
As such, the atom is the basic building block of chemistry. Models of the atom do now:. The first model of the atom was developed through thinking about the smallest particles of matter without experimenting.
Thomson proposed the first of many atomic models to come. 7 October 15 – 18 November 1962) was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory, for which he received the Nobel Prize in Physics in 1922. Rutherford found that the positive charge of an atom and most of its mass were at the center, or nucleus, of an atom.
Democritus and the Atom Even though Democritus was the first to use the word atom he wasn't recognized for it and never had a atomic model or theory. Niels Henrik David Bohr (Danish:. He also discovered that atoms are solid, insdestructable, and unique.
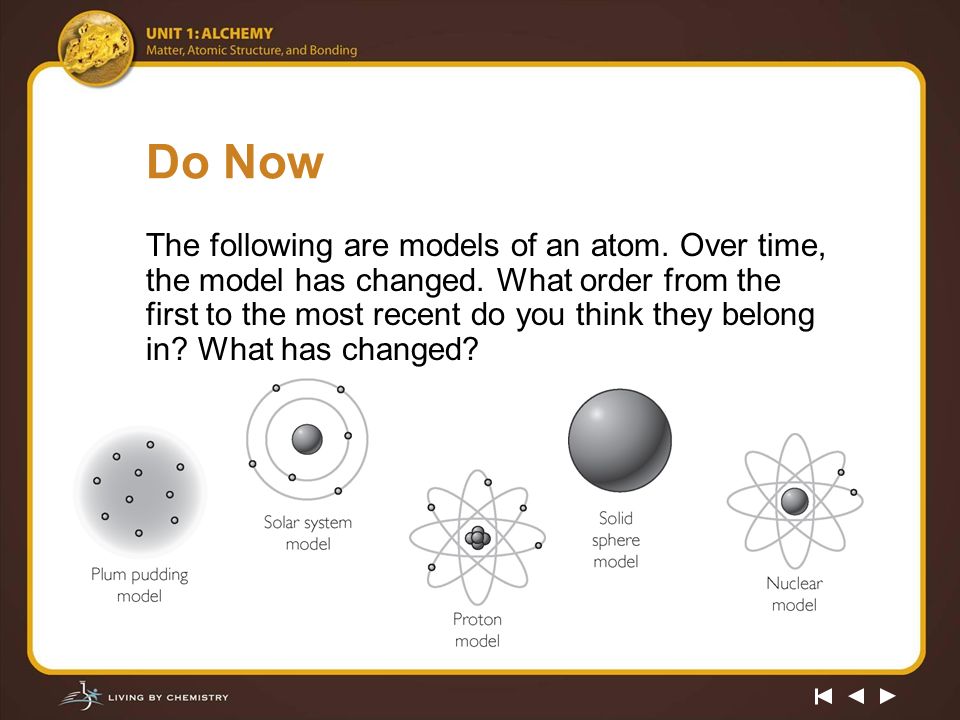
He has images of four models of the atom, but they are not in the correct order. It still has its uses too;. Typical textbook model of an atom Look in an intro, non-science majors textbook and you will probably see a picture like this of the atom.
To the extent that the Schrödinger equation can be solved for more-complex cases, atomic theory is capable of predicting from first principles the. There has been a variety of atomic models throughout history of atomic physics, that refers mainly to a period from the beginning of 19th century to the first half of th century, when a final model of atom which is being used nowadays (or accepted as the most accurate one) was invented.

The History Of The Atomic Model Thomson And The Plum Pudding
3
Q Tbn 3aand9gcqat4ot7tlgmwe 6 N5yqu9c8tgykurdgpatydeoi4 Usqp Cau
First Model Of The Atom のギャラリー

Dublin Schools Lesson Bohr S Model Of The Atom Whose Atomic Model First Accounted For Defined Energy Levels

History Of The Atom Ck 12 Foundation

History Of The Atomic Model Timeline Timetoast Timelines

Building A Simple Model Of The Atom

What Is The Plum Pudding Atomic Model Universe Today

8 2 Quantization Of The Energy Of Electrons Chem 1114 Introduction To Chemistry

Evolution Of The Atomic Structure Timeline Timetoast Timelines

Bohr S Atomic Model Chemistrygod

Models Of The Atom The Atom Siyavula
Write Bohrs Postulates For The Hydrogen Atom Model Class 10 Physics Cbse

Chapters 18 Atoms And The Periodic Table Models Of The Atom 1 Dalton S Model Proposed The First Model Of Atoms In The Early 1800 S Thought Atoms Ppt Download

Plum Pudding Model Of The Atom What Is It Who Discovered It Electrical4u

Models Of The Atom The Atom Siyavula

Dublin Schools Lesson Bohr S Model Of The Atom Whose Atomic Model First Accounted For Defined Energy Levels

Atomic Theory Flashcards Quizlet

Atoms Throughout History Storyboard By Okayz

Bohr Model Description Development Britannica

1 What Democritus Thought Atomic Models
Bohr Model Wikipedia

The First Model Of An Atom Was Given By

The Plum Pudding Model How A Flawed Idea Was Instrumental In Our Understanding Of The Atom

Atomic Model Timeline Timetoast Timelines

Bohr S Model Of An Atom With Postulates And Limitations Byju S

Atomic Structure Ppt Video Online Download
Atomic Physics

Dublin Schools Lesson Bohr S Model Of The Atom Whose Atomic Model First Accounted For Defined Energy Levels
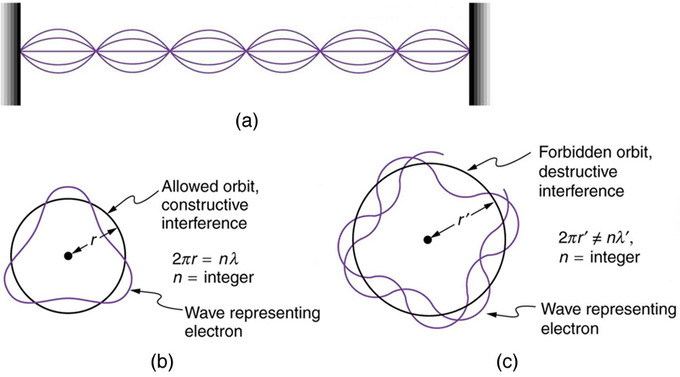
Atomic Structure

A Tale Of Two Models American Welding Society Education Online

Reading Exercise The History Of The Atomic Model A Tang Of Science
Bohr Model Of The Atom Overview And Examples

Models Of The Atom The Atom Siyavula

The Early Atom Boundless Physics
The Early Atom Boundless Physics

Solved Workshop Tutorials For Physics Qr5 Atomic Structu Chegg Com
Www Sisd Net Cms Lib Tx Centricity Domain 1297 The History Of The Atom Notes Condensed Pdf
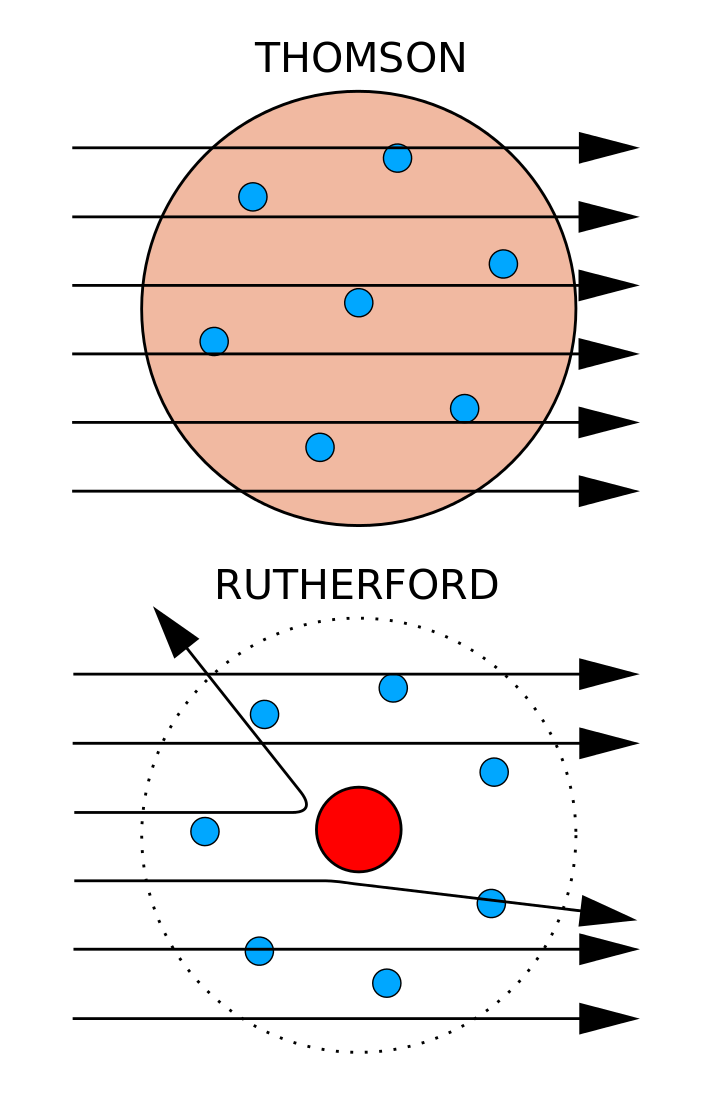
Experimental Evidence For The Structure Of The Atom

Bohr Model Description Development Britannica
Q Tbn 3aand9gcryadcko5qh56frjjrqxhnao2jw1gwoufhwij 0qwsubfwr7fyj Usqp Cau

Niels Bohr
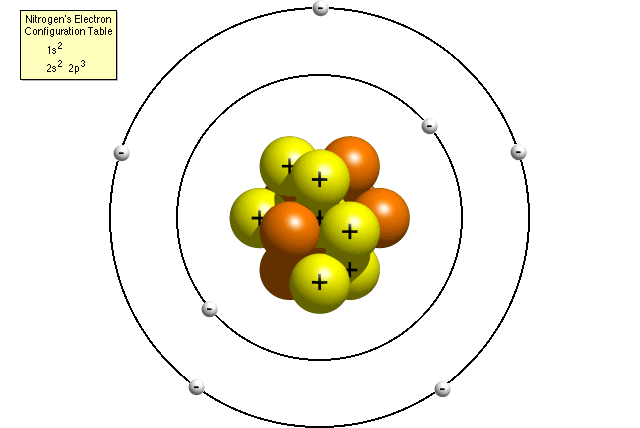
Questions And Answers How Do I Make A Model Of An Atom

Atoms By Julia Russell

The Diagram Shows Niels Bohr S Model Of An Atom What Happens When The Electron Moves From The First Brainly Com
Rutherford Model Wikipedia

Orbitals And The Octet Rule
Atoms Elements And The Periodic Table Ppt Download
Bohr Model Of The Atom Overview And Examples
The Standard Model Of The Atom Ppt Download
Click And Learn Atomic Theories
Bohr S Hydrogen Atom Chemistry Libretexts
Bohr S Model Of Hydrogen Article Khan Academy

History Of The Atom The Idea Of The Atom Has Been Around Since 400
Atomic Theory

Chapter 12 Atoms
Ernest Rutherford History Of The Atomic Theory

Atomic Model Timeline Timetoast Timelines

Morgan S Chemistry Blog Atomic Models

Q Tbn 3aand9gcqx0q13d2srx38t9bdplhd3vqk Eboyxg7ldq Usqp Cau
The History Of The Atomic Model Thomson And The Plum Pudding

Rutherford Model Definition Facts Britannica

History Of The Atom Ck 12 Foundation

Models Over Time The Atom
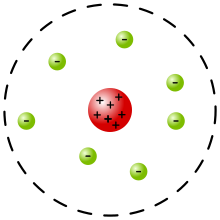
Rutherford Model Wikipedia

Timeline Structural Theory Chemogenesis Chemistry Teaching Chemistry Chemistry Classroom

Bohr S Model Of Hydrogen Article Khan Academy
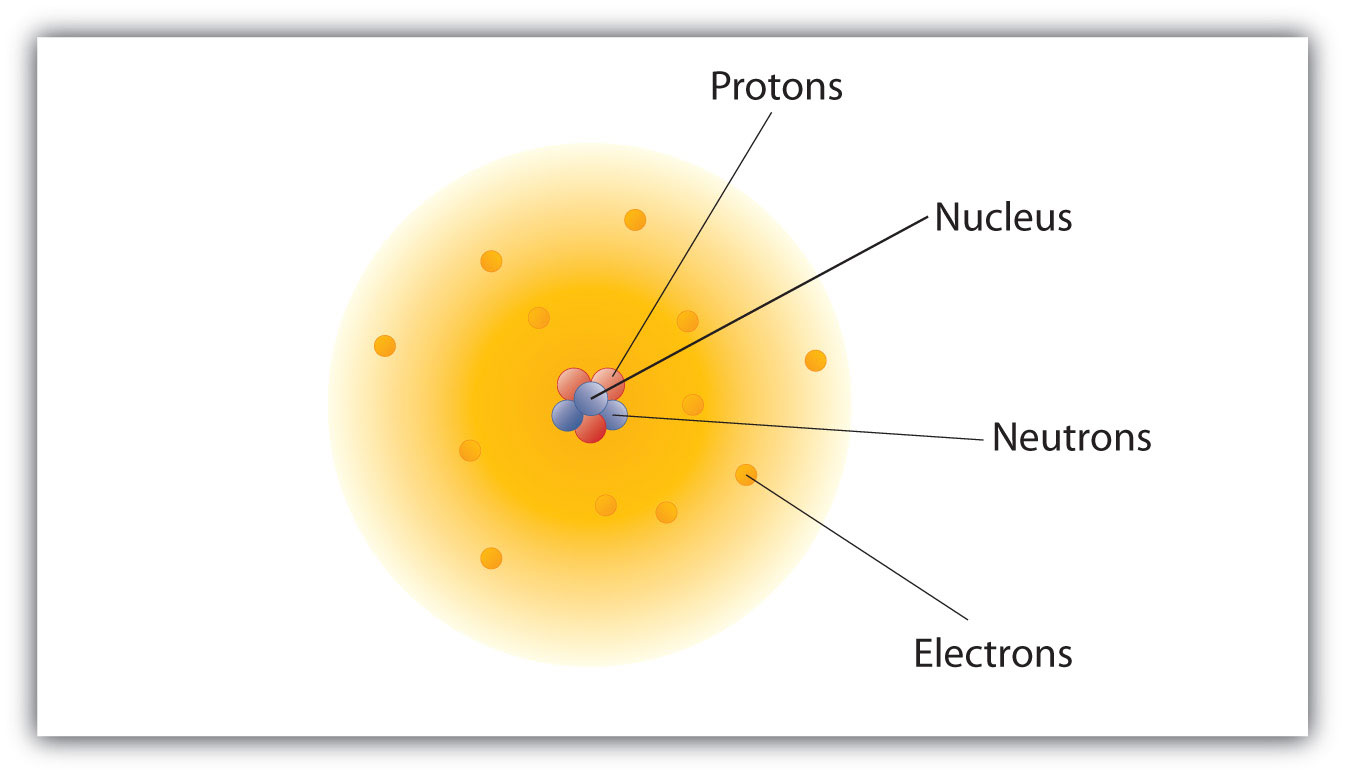
Atomic Theory

A Timeline Of Atomic Models Did You Know That The Atomic Model Has By Intlink Education Medium

Development Of Atomic Theory
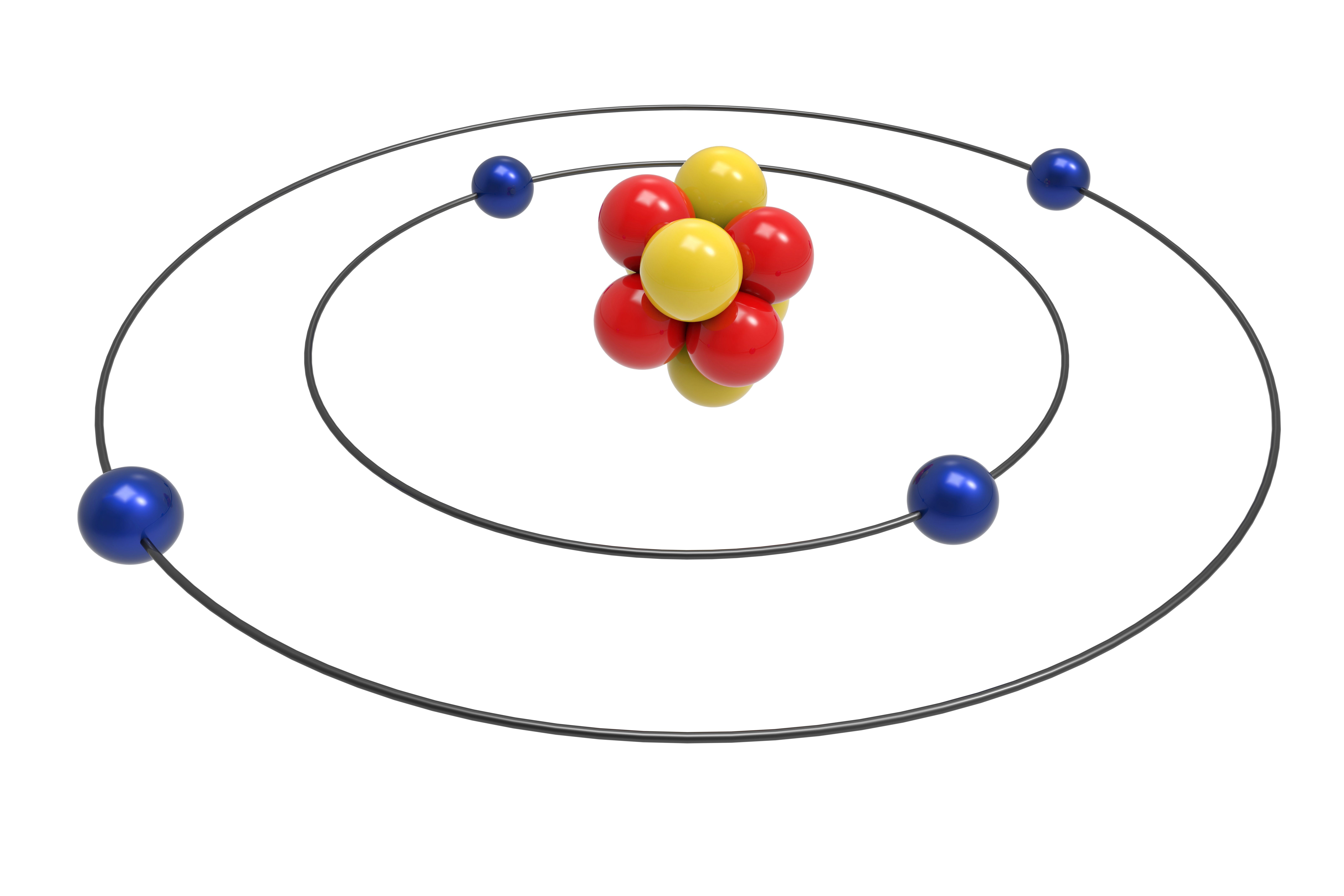
Five Types Of Atomic Models
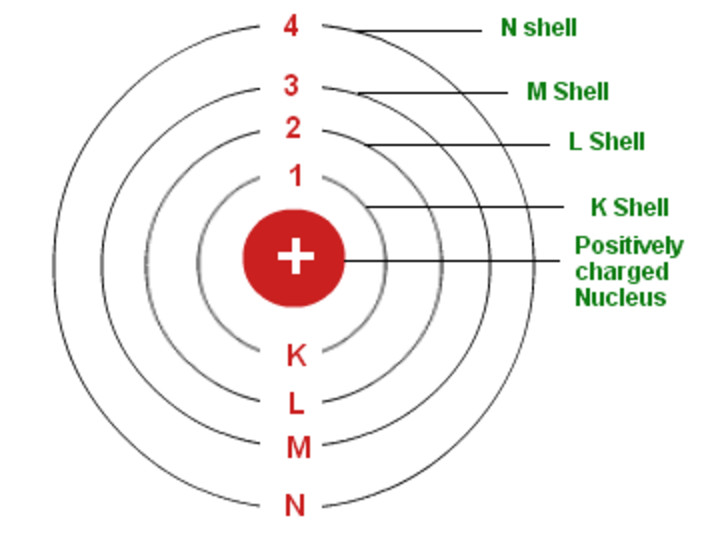
Bohr S Model Of An Atom Chemistry Class 11 Structure Of Atom

2 1e Electron Shells And The Bohr Model Biology Libretexts
Www Sisd Net Cms Lib Tx Centricity Domain 1297 The History Of The Atom Notes Condensed Pdf
Q Tbn 3aand9gctrqcw Tozvpm19z6ohcrbrxsbrdsedtjqjsv Uwj7mnm Ixhfe Usqp Cau

Atomic Theory Flashcards Quizlet

Bohr S Model Of An Atom With Postulates And Limitations Byju S

Atomic Theory Wikipedia
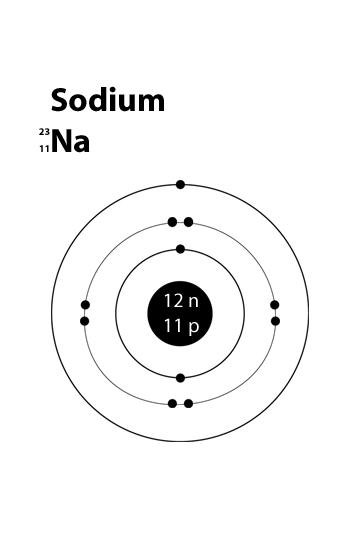
How Can I Make A Sodium Atom Model Socratic

Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com

Models Of The Atom

Quantum Model Of The Atom

First Light Reionization Hydrogen Atom Atom Model Atom Model Project
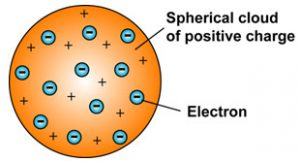
J J Thomson Model Of An Atom Class 9 Structure Of An Atom
Who Saw Atoms And When Quora

Bohr Model Of The Atom

Atoms
How Did The Atomic Model Changed Over Time

Bohr Atomic Model
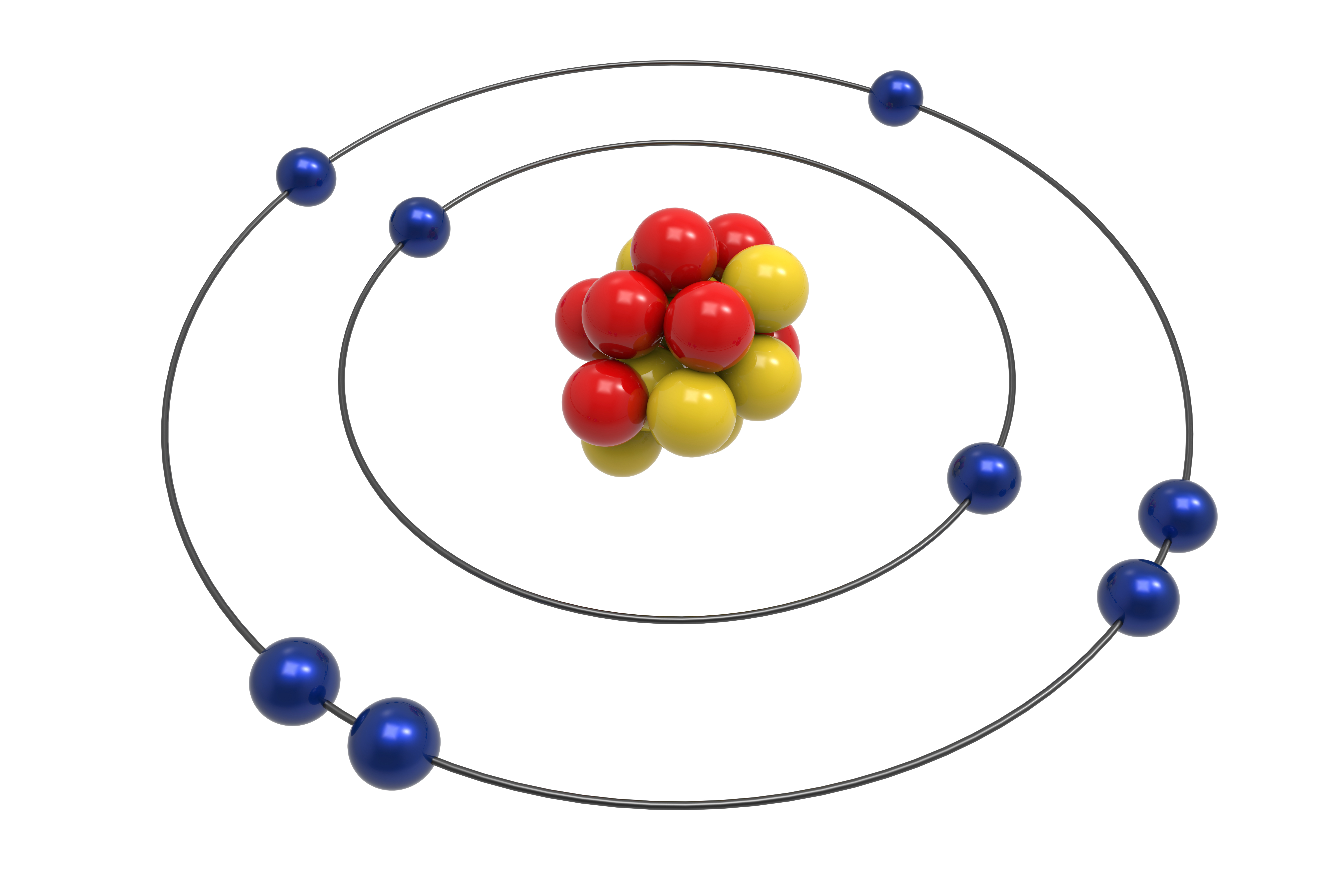
What Are The 4 Atomic Models
Atomic Models Wikilectures

How Our Model Of Atoms Has Changed Over Number Of Times Since We First Conceived It Physics
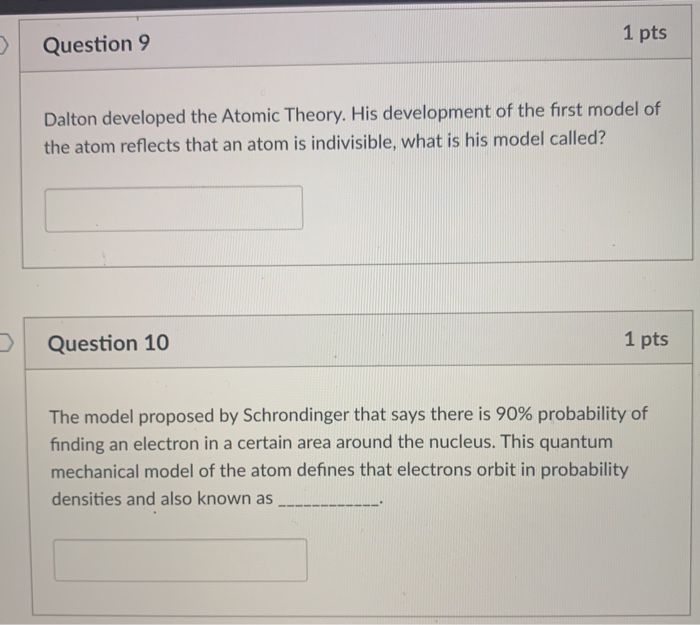
Solved 1 Pts Question 9 Dalton Developed The Atomic Theor Chegg Com

Bohr Model Of First 18 Elements Of The Periodic Table 11 Download Scientific Diagram

Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com

History Of The Atom Our Lesson Shows The Major Discoveries That Helped Develop The Modern Atomic Model From D Atomic Theory Dalton Atomic Model Gcse Chemistry

The First Model Of An Atom Was Given By

Early Atomic Theory Dalton Thomson Rutherford And Millikan Video Lesson Transcript Study Com

The History Of The Atom Theories And Models Compound Interest

Plum Pudding Model Of The Atom What Is It Who Discovered It Electrical4u

Atom Model Universe Today

A Timeline Of Atomic Models Did You Know That The Atomic Model Has By Intlink Education Medium

Dalton S Atomic Model Brilliant Math Science Wiki

Atomic Models Thomson S Atomic Model And Rutherford S Atomic Model




