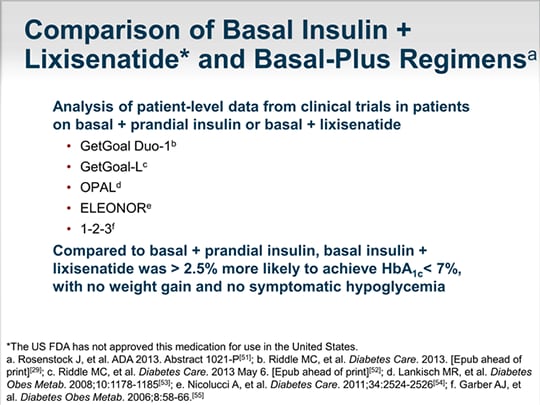
Getgoal Duo 1
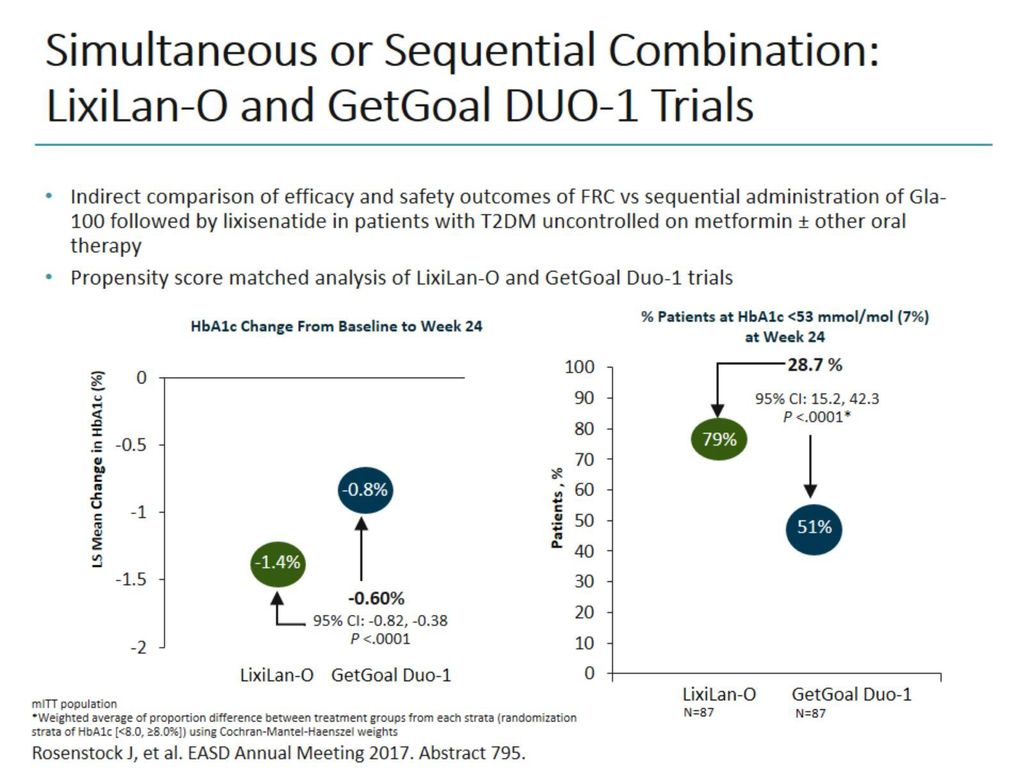
To conduct two exploratory analyses to compare indirectly the efficacy and safety of simultaneous administration of insulin glargine 100 U (iGlar) and the glucagon-like peptide-1 receptor agonist (GLP-1RA) lixisenatide (Lixi) as a single-pen, titratable, fixed-ratio combination (iGlarLixi LixiLan trials) vs sequential administration of iGlar + Lixi (GetGoal Duo trials) in people with.

Getgoal duo 1. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. Results showed that lixisenatide caused mild and transient nausea and vomiting, the most common adverse events, and a limited additional or comparable risk of hypoglycemia. In GetGoal DUO 2, the head-to-head comparison was between lixisenatide 1/d vs glulisine either 1/d (at the main meal, basal-plus) or 3/d (basal-bolus).
OBJECTIVE To provide evidence-based options on how to intensify basal insulin, we explored head-to-head prandial interventions in overweight patients with type 2 diabetes inadequately controlled on basal insulin glargine with or without 1–3 oral antidiabetic agents (OADs). October 12 12:00 - 13:00. After a 12-week run-in phase in which insulin glargine was initiated, patients with A1C ≥7% were randomized to μg lixisenatide ( n = 223) or placebo ( n = 223) for 24 weeks while continuing on insulin glargine.
NCT (GetGoal Duo-2 trial). RIDDLE THOMAS FORST RONNIE ARONSON FRCPC FACE LEOBARDO SAUQUE-REYNA ELISABETH SOUHAMI LOUISE SILVESTRE LIN PING JULIO ROSENSTOCK A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) OBJECTIVEdWhen oral therapy for type 2 diabetes is ineffective, adding basal insulin improves glycemic control. The trials were conducted between July 08 and August 11 across 25 countries (the number of countries and enrolment/completion dates varied by trial).
Further results are expected in 12. To date, GetGoal-X, GetGoal-L, GetGoal-L Asia, GetGoal-Mono, GetGoal-S, GetGoal-F1 and GetGoal Duo 1 (also known as EFC*) have reported positive top-line results supporting potential efficacy and safety for lixisenatide. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:.
Riddle MC, Forst T, Aronson R et al. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). These patients were on existing basal insulin therapy with or without a SU.
Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. 21 – 26 The GetGoal-X trial, which compared lixisenatide with twice-daily exenatide, resulted in weight loss of 2.96 kg and 3.98 kg, respectively. GetGoal Duo 1 was a randomized, double-blind, multicenter study assessing the efficacy and safety of lixisenatide compared to placebo in combination with Lantus (insulin glargine;.
Lixisenatide activates the GLP-1 receptor and thereby exercises the range of physiological effects generated by GLP-1, which consist of increased insulin secretion, inhibition of glucagon secretion, and decreased gastrointestinal motility alongside the promotion of. The GetGoal-Duo 1 study ePoster # 807 Session:. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1).
Try Duo for web. GETGOAL GLP-1 agonist AVE0010 in patients with type 2 diabetes mellitus for glycaemic control and safety evaluation (lixisenatide, µg once daily), GLP-1 RA glucagon-like peptide-1 receptor agonist,. Advancing Basal Insulin Glargine with Prandial Lixisenatide QD vs Insulin Glulisine QD or TID in T2DM:.
A 24-week, randomized, placebo-controlled study. Sanofi) and OADs. PS 063 GLP-1 based therapies Berlin 12 Poster Hall 3.
A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Secondary Endpoints Change in 2-hr PPG Change in Body Weight. Sanofi announced positive results from its Phase 3, GetGoal Duo 1 study of Lyxumia (lixisenatide) in combination with Lantus (insulin glargine;.
Efficacy and safety of once-daily lixisenatide added on to titrated glargine plus oral agents in type 2 diabetes:. Lantus(R) is the No.1 leading basal insulin product in the world, and the results from GetGoal Duo 1 show that adding lixisenatide to treatment with Lantus(R) can offer significant benefits to patients." "Lixisenatide is a promising new GLP-1 agonist with a mode of action which complements that of basal insulin. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:.
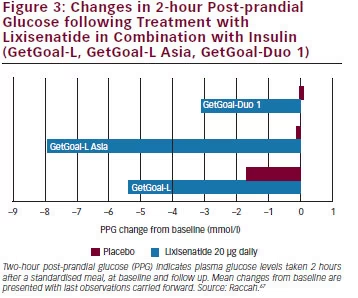
A 24- week, Randomized, Placebo-Controlled Study (GetGoal Duo 1).” Diabetes Care 36.9 (13):. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). GetGoal Duo 1 and GetGoal-L both achieved the primary efficacy endpoint of HbA1c improvement with an associated significant reduction in PPG.
Riddle MC, Forst T, Aronson R, et al. Presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia PA, 8-12 June 12 (Abstract 62-OR). Riddle MC, Forst T, Aronson R, et al.
In the GetGoal Duo 1, randomized, double-blind, multicenter study, 8 insulin-naïve patients were treated with insulin glargine (Lantus), which was titrated to reach a target fasting plasma glucose (FPG) of 80-100 mg/dL for a 12-week run-in phase period. "During a 12-week run-in phase, 8 insulin-naive patients were. Epub 16 May 23.
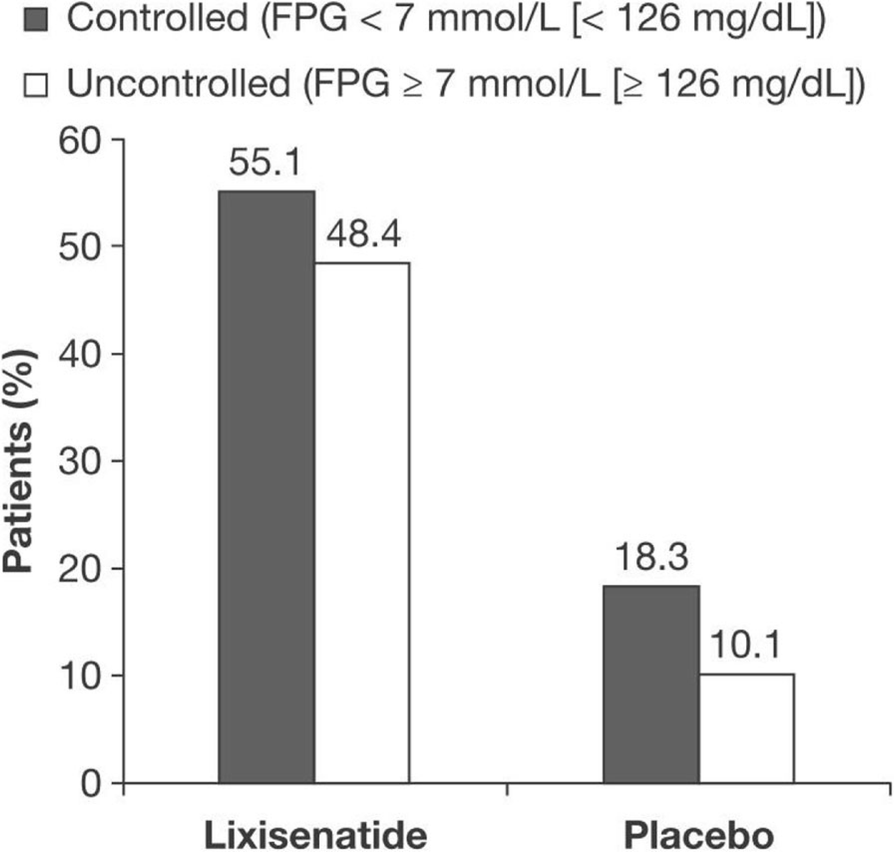
The originality of GetGoal DUO 2 is the comparison between add-on of lixisenatide and active treatment (prandial insulin). Lixisenatide improved HbA1c, weight with standard GLP-1 safety profile PHILADELPHIA — Once-daily lixisenatide, an investigational GLP-1 agonist, was associated with significant HbA1c. Patients in GetGoal-Duo 1 were from 25 countries and were inadequately controlled (HbA1c 53–86 mmol/mol 7.0–10.0%) on existing OAD therapy.
It’s free, simple and works on Android phones, iPhones, tablets, computers, and smart displays, like the Google Nest Hub Max. In GetGoal Duo 1, lixisenatide in combination with insulin glargine achieved the primary study endpoint of significantly reducing HbA1c with a significant improvement in 2-hour post-prandial. Patients in GetGoal-Duo 1 were from 25 countries and were inadequately controlled (HbA1c 53–86 mmol/mol 7.0–10.0%) on existing OAD therapy.
Patients in GetGoal-L Asia were from Japan, Republic of Korea, Taiwan, and the Philippines. So far, GetGoal-X, GetGoal-L, GetGoal-L Asia, GetGoal-Mono, GetGoal-S, GetGoal-F1 and GetGoal Duo 1 have reported positive top-line results. The GetGoal program started in May 08 and has enrolled more than 4,500 patients.
The GetGoal Duo-1 trial was the first study to assess the efficacy of lixisenatide in combination with optimally titrated basal insulin in patientswith type2 diabe-tesuncontrolledonOADswhowerenewly initiatinginsulinglargine.Signi ficantreduc-tions in HbA 1c to 7% (53 mmol/mol) and marked PPG reductions were achieved. The GetGoal program was initiated in May 08. Sanofi) for the treatment of patients with type 2.
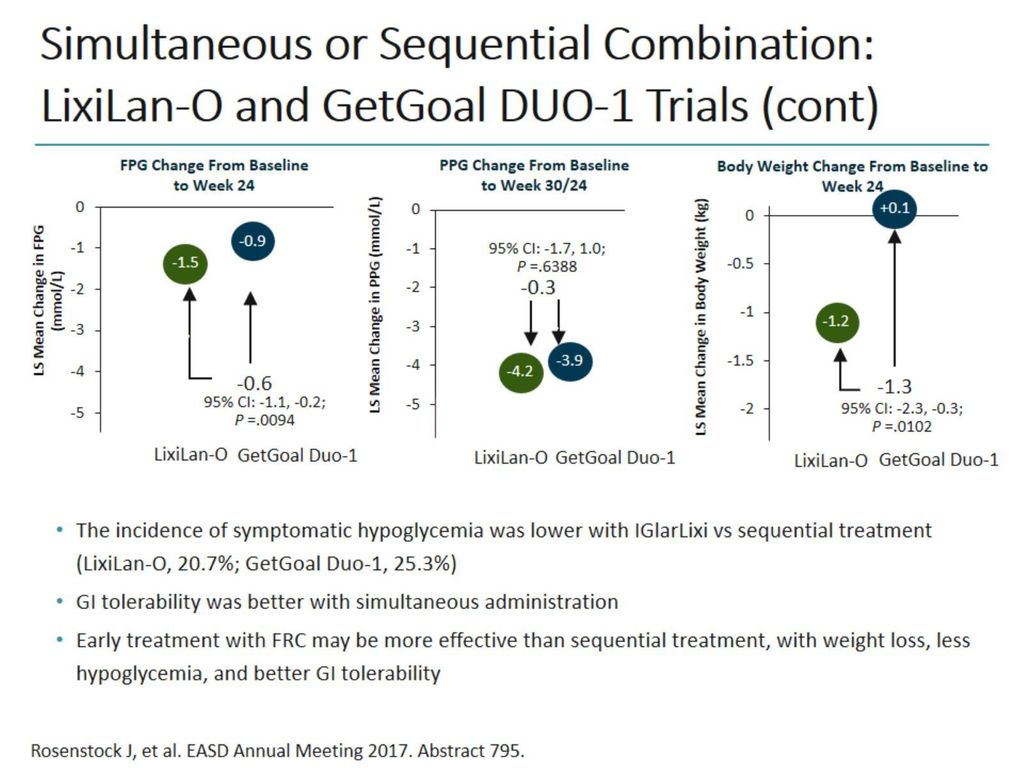
Therefore, propensity score matching was used to indirectly compare simultaneous administration of iGlarLixi in the LixiLan-O trial (n=469) with sequential therapy, starting with initial insulin glargine 100 U/mL therapy for 12 weeks, followed by addition of lixisenatide in the GetGoal Duo-1 trial in patients with Type 2 diabetes mellitus who. GetGoal DUO 2 follows the studies GetGoal-L and GetGoal DUO 1 , which have both explored the efficacy and safety of once-a-day add-on of lixisenatide to basal insulin at fixed dose , or to insulin glargine with continuing titration as compared to placebo. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1).
Six randomized, placebo-controlled studies of lixisenatide µg once daily were included in this analysis:. Duo is the highest quality 1 video calling app. Rosenstock J, Forst T, Aronson R, et al.
Julio Rosenstock 1 , Bruno Guerci 2 , Markolf Hanefeld 3 , Sandro Gentile 4 , Ronnie Aronson 5 , Francisco J Tinahones 6 , Christine Roy-Duval 7 , Elisabeth Souhami 7 , Marek Wardecki 8 , Jenny Ye 9 , Riccardo Perfetti 9 , Simon Heller 10 , GetGoal Duo-2 Trial Investigators. GetGoal Duo 1 is a randomized, double-blind, multicenter study, assessing the efficacy and safety of lixisenatide, compared to placebo, in combination with insulin glargine and OADs (mostly metformin). Plain language summary available for this article.
The GetGoal Duo 1 study evaluated once-daily lixisenatide added on to titrated glargine plus oral agents in type 2 diabetes. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. The GetGoal-Duo 1 study assessed the complementary action of lixisenatide and insulin glargine in patients with type 2 diabetes failing on oral antidiabetes medication.
Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either as Basal-Plus or Basal-Bolus in Type 2 Diabetes:. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). The GetGoal Duo-2 Trial.
Change in 2-h PPG and glucose. GETGOAL-L-Asia GETGOAL-Duo-1 Cardiovascular outcome trials for GLP-1 RAs. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1).
A two-step dosage increase was used with both placebo and lixisenatide (10 μg for 1 week, 15 μg for 1 week, and then -μg maintenance dosage if tolerated), with injections self-administered by participants ≤1 h before breakfast. Again, as in the 4B, body weight and hypoglycemia incidence. In conclusion, 1) GLP-1-(7-36) amide or -(7-37) inhibits gastric emptying also in normal subjects, 2) physiological doses (0.4 pmol.kg-1.min-1) still have a significant effect, 3) despite the.
RESEARCH DESIGN AND METHODS Patients were randomized to lixisenatide once daily or insulin glulisine given once or. Riddle, Mattew C.et al. The abstract is titled:.
Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. For Type 2 diabetes patients treated with basal. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1).
GetGoal-M, GetGoal-S) or in combination with basal insulin (GetGoal-L, GetGoal-Duo-1 and GetGoal-L-Asia). Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, Ping L, Rosenstock J. Once-daily lixisenatide added on to consistently titrated insulin glargine plus oral agents in type 2 diabetes:.
-1 0 1 GetGoal-L1 GetGoal-L Asia2 GetGoal-Duo 13 e ) ~16.0 (2 mg/dL) 17.8 (3.4 mg/dL) ~13.0 (234 mg/dL) Baseline PPG Lixisenatide Added to Background Insulin:. Lixisenatide as monotherapy (GetGoal-Mono), as add-on to oral antidiabetic drugs (OADs;. The GetGoal Duo-2 Evidence-Based Trial (NCT).
Efficacy and Safety of iGlarLixi, Fixed-Ratio Combination of Insulin Glargine and Lixisenatide, Compared with Basal-Bolus Regimen in Patients with Type 2 Diabetes:. During the 12-week run-in phase, 8 insulin-naive patients were treated with insulin glargine, which was titrated to reach a target fasting. Efficacy and Safety of Lixisenatide Versus Placebo on Top of Basal Insulin and/or Oral Antidiabetic Treatment in Older Type 2 Diabetic Patients (GetGoal-O) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
MC Riddle, T Forst, R Aronson, et al.Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. Like in 4B, in GetGoal DUO 2 the A1C decreased to similar values with lixisenatide or glulisine 1/d (~7.2%), or glulisine 3/d (~7.0%). Efficacy and Safety of Lixisenatide Versus Insulin Glulisine on Top of Insulin Glargine With or Without Metformin in Type 2 Diabetic Patients (GetGoal-Duo-2) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
Adjustment of dosage of insulin glargine. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). “Adding Once – Daily Lixisenatide for Type 2 Diabetes Inadequately Controlled With Newly Initiated and Continuously Titrated Basal Insulin Glargine:.
If present, SU therapy was discontinued, and patients were initiated on basal insulin therapy with or without MET or a TZD during the run-in phase. Patients were randomized to receive lixisenatide or placebo 1:1 in GetGoal-Duo1 and GetGoal-L-Asia, and 2:1 in GetGoal-L. Prandial Options to Advance Basal Insulin Glargine Therapy:.
"The results from the GetGoal Duo-2 study reconfirm the therapeutic benefits of Lyxumia(R) as a novel prandial GLP-1 agonist. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. Propensity Score Matched Analysis.
Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. The glucagon-like peptide (GLP)-1 receptor agonist lixisenatide (Lyxumia®) was approved for marketing by the European Medicines Agency in February 13 and has been evaluated in a clinical study program called GetGoal. In the GetGoal-Duo-1 trial, both groups gained weight, but patients in the lixisenatide arm gained less than those in the control arm (0.3 kg versus 1.2 kg, respectively;.

Composite Of A Glycated Haemoglobin Hba1c B Fasting Plasma Glucose Download Scientific Diagram

Session Two Changing The Type 2 Diabetes Mellitus Management Paradigm With Fixed Ratio Combinations European Medical Journal
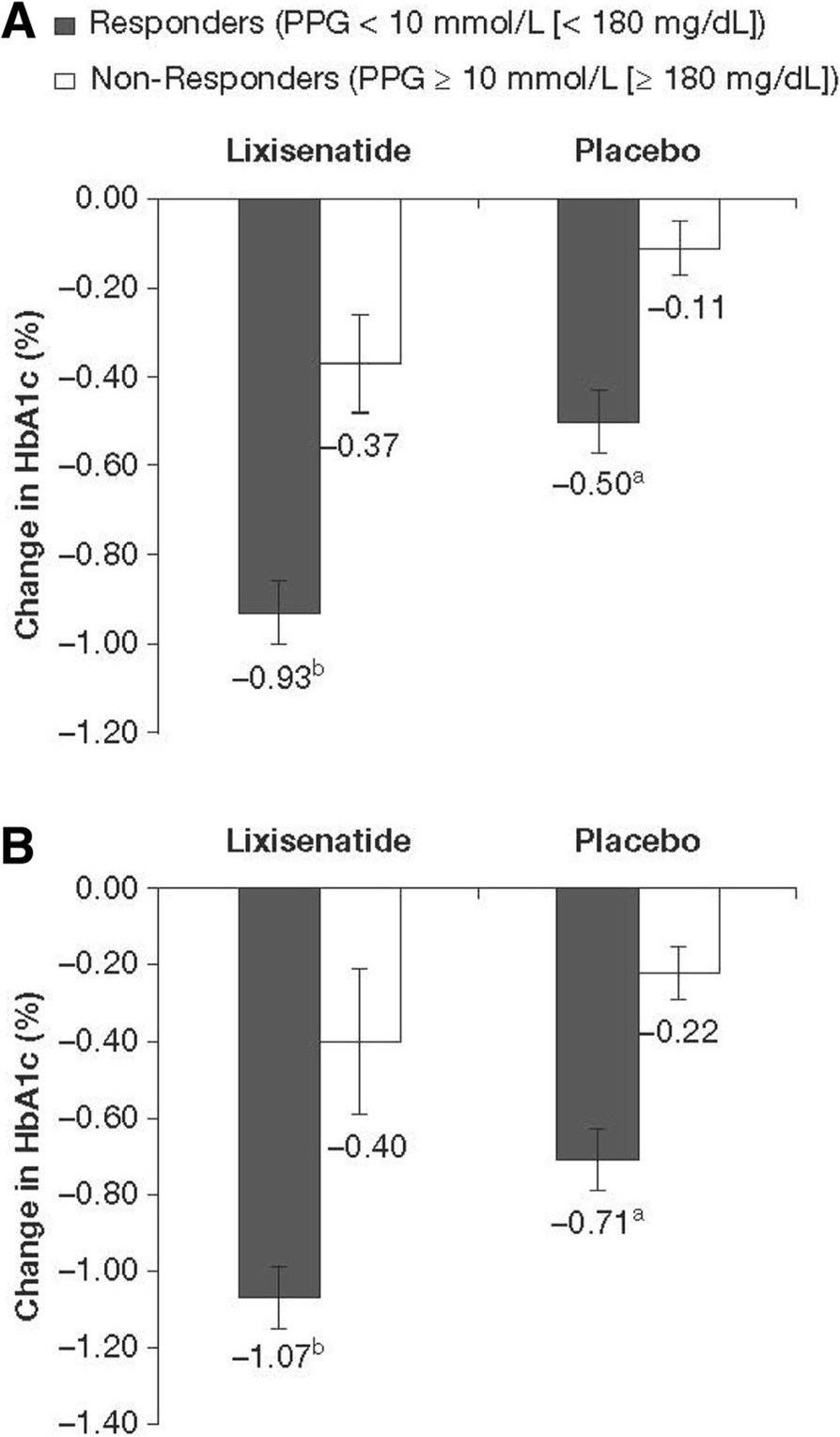
Achieving Postprandial Glucose Control With Lixisenatide Improves Glycemic Control In Patients With Type 2 Diabetes On Basal Insulin A Post Hoc Analysis Of Pooled Data Springerlink
Getgoal Duo 1 のギャラリー
Www Sanofi Com Media Project One Sanofi Web Websites Global Sanofi Com Home En Investors Docs I P Ir Call Ada15 Final Pdf La En Hash 53cad627a4edf9ebcb3ed
Insulin Glp 1 Agonist Combinations Ppt Download
Combination Therapy With Insulins And Glp 1 Receptor Agonists

Initiating Injectable Treatment Of Type 2 Diabetes A Focus On Glucagon Like Peptide 1 Receptor Agonists Consultant360

Pdf Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial Semantic

Pdf The Efficacy And Safety Of Lixisenatide In A Predominantly Asian Population With Type 2 Diabetes Insufficiently Controlled With Basal Insulin The Getgoal L C Randomized Trial

A Systematic Review And Meta Analysis Of The Efficacy Of Lixisenatide In The Treatment Of Patients With Type 2 Diabetes Schmidt 14 Diabetes Obesity And Metabolism Wiley Online Library
Lixisenatide A New Glucagon Like Peptide 1 Receptor Agonist In The Treatment Of Type 2 Diabetes Touchendocrinology
Gale Academic Onefile Document Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1

Intensification Of Basal Insulin Therapy With Lixisenatide In Patients With Type 2 Diabetes In A Real World Setting The Basal Lixi Study Sciencedirect
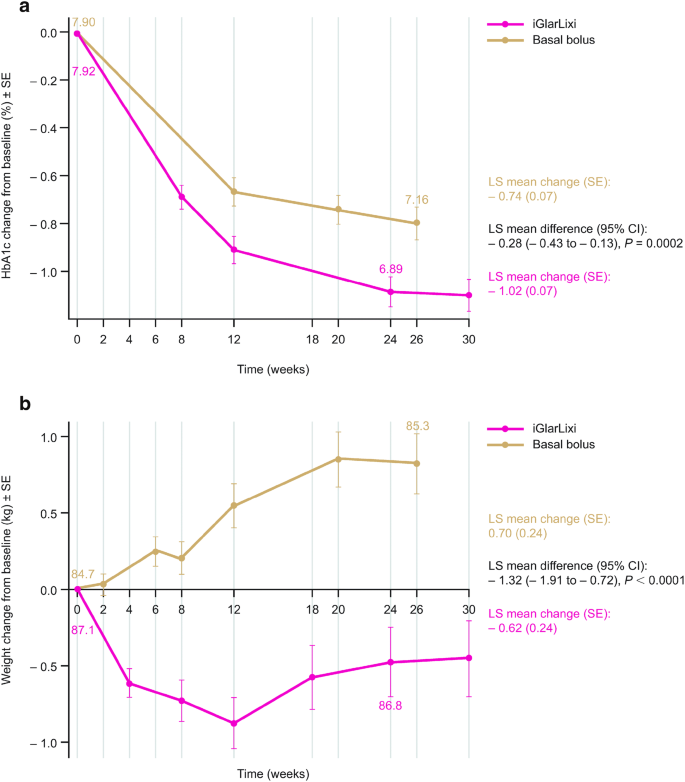
Efficacy And Safety Of Iglarlixi Fixed Ratio Combination Of Insulin Glargine And Lixisenatide Compared With Basal Bolus Regimen In Patients With Type 2 Diabetes Propensity Score Matched Analysis Springerlink

Is There A Justification For Classifying Glp 1 Receptor Agonists As Basal And Prandial Topic Of Research Paper In Clinical Medicine Download Scholarly Article Pdf And Read For Free On Cyberleninka Open

Propensity Score Matched Comparative Analyses Of Simultaneously Administered Fixed Ratio Insulin Glargine 100 U And Lixisenatide Iglarlixi Vs Sequential Administration Of Insulin Glargine And Lixisenatide In Uncontrolled Type 2 Diabetes Abstract
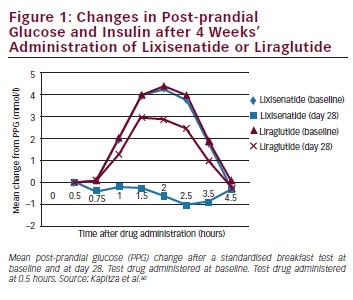
Lixisenatide A New Glucagon Like Peptide 1 Receptor Agonist In The Treatment Of Type 2 Diabetes Touchendocrinology

Efficacy And Safety Of Lixilan A Titratable Fixed Ratio Combination Of Lixisenatide And Insulin Glargine Versus Insulin Glargine In Type 2 Diabetes Inadequately Controlled On Metformin Monotherapy The Lixilan Proof Of Concept Randomized Trial
Gale Academic Onefile Document Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1
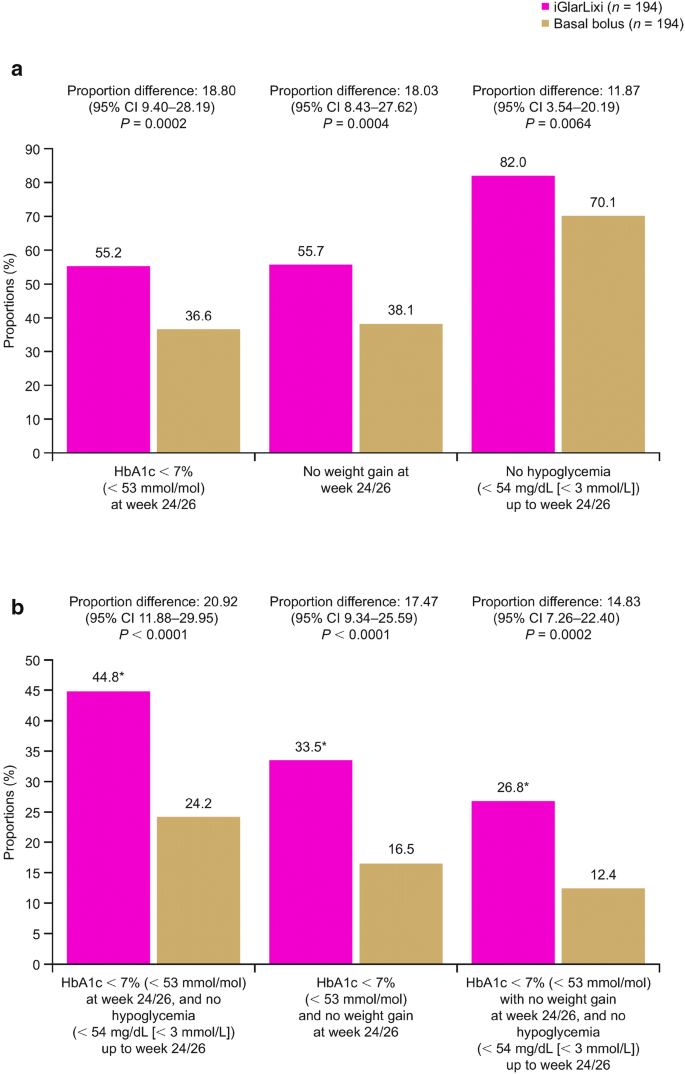
Efficacy And Safety Of Iglarlixi Fixed Ratio Combination Of Insulin Glargine And Lixisenatide Compared With Basal Bolus Regimen In Patients With Type 2 Diabetes Propensity Score Matched Analysis Springerlink

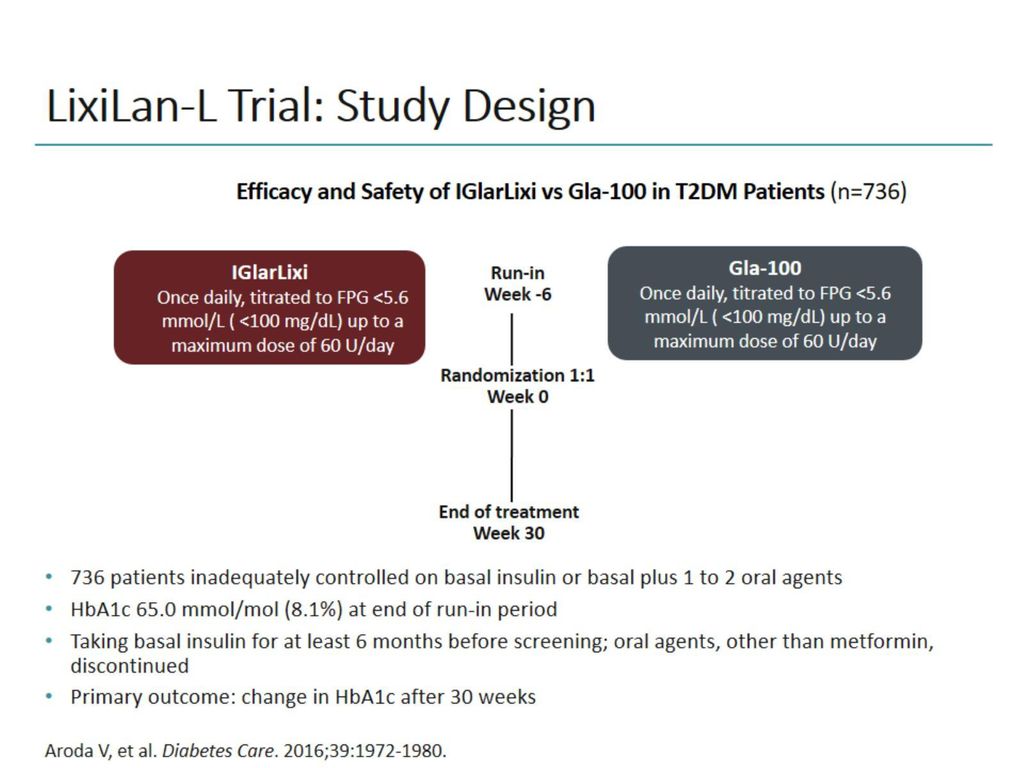
Study Design Of Getgoal Duo 1 16 Download Scientific Diagram

The Clinical Development Program Of Lixisenatide A Once Daily Glucagon Like Peptide 1 Receptor Agonist Abstract Europe Pmc

Full Text Lixisenatide As Add On Therapy To Basal Insulin Dddt

The Clinical Development Program Of Lixisenatide A Once Daily Glucagon Like Peptide 1 Receptor Agonist Abstract Europe Pmc
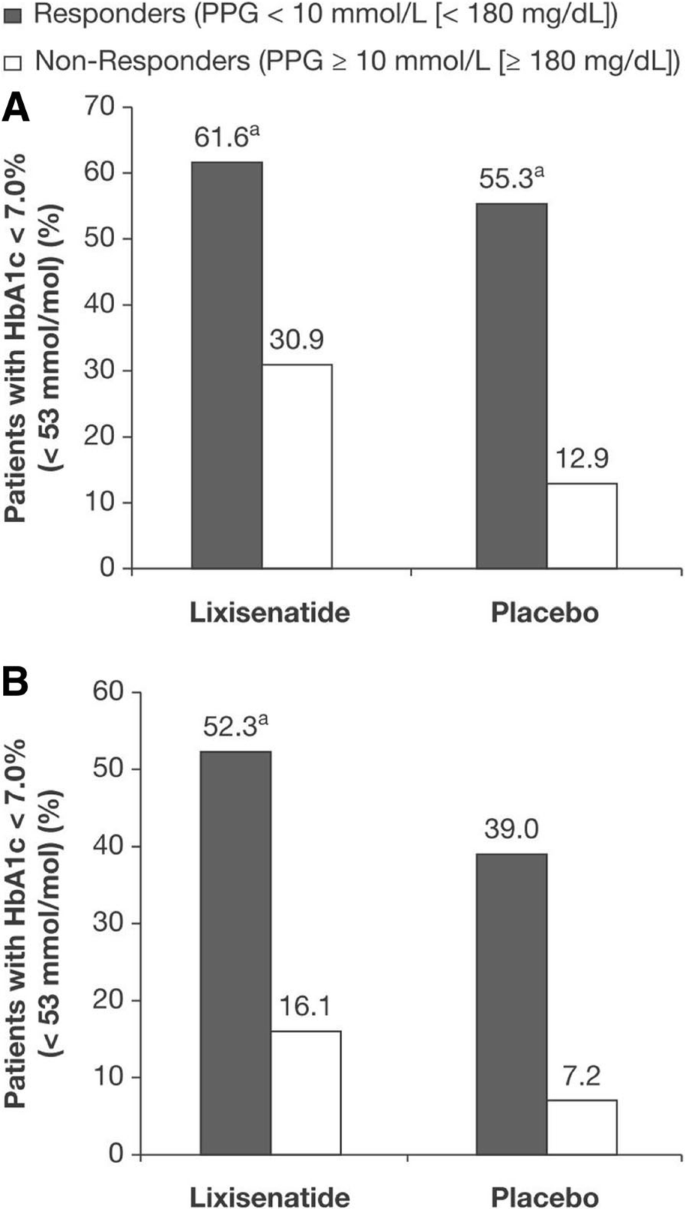
Achieving Postprandial Glucose Control With Lixisenatide Improves Glycemic Control In Patients With Type 2 Diabetes On Basal Insulin A Post Hoc Analysis Of Pooled Data Clinical Diabetes And Endocrinology Full Text
2

Lyxumia Lixisenatide In Combination With Basal Insulin Plus Oral Anti Diabetics Significantly Reduced Hba1c And Post Prandial Glucose
Full Article Lixisenatide As Add On To Insulin Glargine For The Treatment Of Type 2 Diabetes Mellitus

Supplemental Materials For Glucagon Like Peptide 1 Receptor Agonist And Basal Insulin Combination Treatment For The Management Of Type 2 Diabetes A Systematic Review And Meta Analysis The Lancet

Switching From Insulin Bolus Treatment To Glp 1 Ras Added To Continued Basal Insulin In People With Type 2 Diabetes On Basal Bolus Insulin Diabetes Care

Full Text Lixisenatide As Add On Therapy To Basal Insulin Dddt
Results Clinical Review Report Lixisenatide Adlyxine Ncbi Bookshelf

Benefits Of Lixilan A Titratable Fixed Ratio Combination Of Insulin Glargine Plus Lixisenatide Versus Insulin Glargine And Lixisenatide Monocomponents In Type 2 Diabetes Inadequately Controlled On Oral Agents The Lixilan O Randomized Trial

The Clinical Development Program Of Lixisenatide A Once Daily Glucagon Like Peptide 1 Receptor Agonist Topic Of Research Paper In Clinical Medicine Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science

Table 3 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar

Reviewer Worksheets Pharmacoeconomic Review Report Lixisenatide Adlyxine Ncbi Bookshelf

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine Diabetes Care

New Forms Of Insulin And Insulin Therapies For The Treatment Of Type 2 Diabetes The Lancet Diabetes Endocrinology

Propensity Score Matched Comparative Analyses Of Simultaneously Administered Fixed Ratio Insulin Glargine 100 U And Lixisenatide Iglarlixi Vs Sequential Administration Of Insulin Glargine And Lixisenatide In Uncontrolled Type 2 Diabetes Rosenstock
2

Pdf Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1

Pdf Injectable Coformulations In Diabetology Semantic Scholar

A Systematic Review And Meta Analysis Of The Efficacy Of Lixisenatide In The Treatment Of Patients With Type 2 Diabetes Schmidt 14 Diabetes Obesity And Metabolism Wiley Online Library
Http Journals Sagepub Com Doi Pdf 10 1177

Challenges And Opportunities In The Treatment Of Type 2 Diabetes Nancy A Thornberry Pdf Free Download

Once Daily Lixisenatide Added On To Consistently Titrated Insulin Glargine Plus Oral Agents In Type 2 Diabetes The Getgoal Duo 1 Study Virtual Meeting Easd
Simultaneous Vs Sequential Combination Of Insulin Glargine And Lixisenatide In Type 2 Diabetes Uncontrolled On Metformin Virtual Meeting Easd

The Role Of Glp 1 Receptor Agonists As Weight Loss Agents In Patients With And Without Type 2 Diabetes Dar 15 Practical Diabetes Wiley Online Library
Insulin Glp 1 Agonist Combinations Ppt Download

Pdf Consensus Recommendations On Glp 1 Ra Use In The Management Of Type 2 Diabetes Mellitus South Asian Task Force Semantic Scholar
Http Www Siditalia It Pdf Relazioni Liguria Congresso 17 Sesti Pdf

Pdf Lixisenatide As Add On Therapy To Basal Insulin Semantic Scholar

Table 4 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar

Once Daily Lixisenatide Added On To Consistently Titrated Insulin Glargine Plus Oral Agents In Type 2 Diabetes The Getgoal Duo 1 Study Virtual Meeting Easd

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine Diabetes Care
Comparison Of Non Insulin Antidiabetic Agents As An Add On Drug To Insulin Therapy In Type 2 Diabetes A Network Meta Analysis Scientific Reports

Initiating Injectable Treatment Of Type 2 Diabetes A Focus On Glucagon Like Peptide 1 Receptor Agonists Consultant360

Study Design Of Getgoal Duo 1 16 Download Scientific Diagram

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine Diabetes Care

Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial Diabetes Care

Study Design Of Getgoal Duo 1 16 Download Scientific Diagram

Table 4 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar

Intensification Of Basal Insulin Therapy With Lixisenatide In Patients With Type 2 Diabetes In A Real World Setting The Basal Lixi Study Sciencedirect

Getgoal Phase Iii Study Programme Download Table
Http Medicoinvestor Com Wp Content Uploads 12 12 Lyxumia Pdf

Efficacy And Safety Of Lixisenatide In Elderly 65 Years And Very Elderly 75 Years Patients With Type 2 Diabetes An Analysis From The Getgoal Phase 3 Programme Virtual Meeting Easd
Http Www Siditalia It Pdf Relazioni Liguria Congresso 17 Sesti Pdf

Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial Diabetes Care
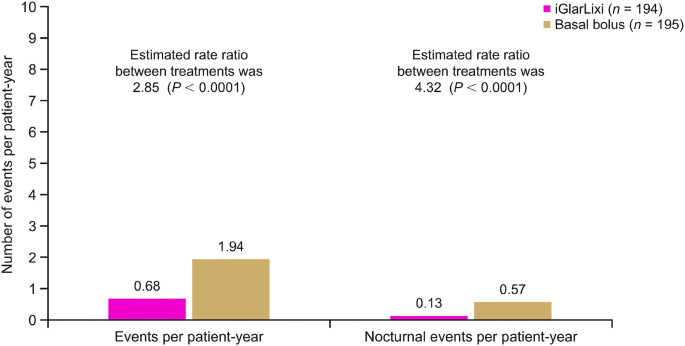
Efficacy And Safety Of Iglarlixi Fixed Ratio Combination Of Insulin Glargine And Lixisenatide Compared With Basal Bolus Regimen In Patients With Type 2 Diabetes Propensity Score Matched Analysis Springerlink
Lixisenatide A New Glucagon Like Peptide 1 Receptor Agonist In The Treatment Of Type 2 Diabetes Touchendocrinology

Table 4 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar
2

Combination Therapy Of Glucagon Like Peptide 1 Receptor Agonists And Insulin For Patients Who Developed Diabetes After Partial Pancreatectomy Kitazawa 16 Journal Of Diabetes Investigation Wiley Online Library

The Rationale For Combining Glp 1 Receptor Agonists With Basal Insulin Cohen 13 Medical Journal Of Australia Wiley Online Library
2
Http Clinical Diabetesjournals Org Content Early 18 02 05 Cd17 0048 Full Text Pdf

Full Text Lixisenatide As Add On Therapy To Basal Insulin Dddt
Add On Options To Basal Insulin Targeting Ppg In Type 2 Diabetes Patients Transcript

Initiating Injectable Treatment Of Type 2 Diabetes A Focus On Glucagon Like Peptide 1 Receptor Agonists Consultant360
Citeseerx Ist Psu Edu Viewdoc Download Doi 10 1 1 1054 3095 Rep Rep1 Type Pdf
Lixisenatide In Type 2 Diabetes Latest Evidence And Clinical Usefulness Abstract Europe Pmc

Predisposing Factors For Any And Major Hypoglycemia With Saxagliptin Versus Placebo And Overall Analysis From The Savor Timi 53 Trial Diabetes Care
Pdf Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1

Table 4 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Ppt Download

Lixisenatide Plus Basal Insulin In Patients With Type 2 Diabetes Mellitus A Meta Analysis Sciencedirect

Pdf Lixisenatide As Add On Therapy To Basal Insulin

Pdf Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1

Composite Of A Glycated Haemoglobin Hba1c B Fasting Plasma Glucose Download Scientific Diagram

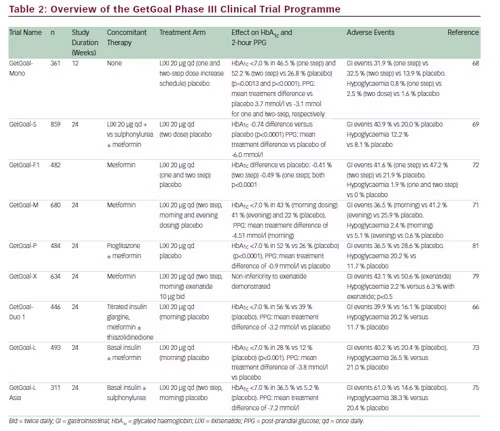
Summary Of The Getgoal Phase Iii Clinical Trial Program With Lixisenatide Download Table

Lixisenatide Plus Basal Insulin In Patients With Type 2 Diabetes Mellitus A Meta Analysis Sciencedirect

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine Diabetes Care

Pdf Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial Semantic

Session Two Changing The Type 2 Diabetes Mellitus Management Paradigm With Fixed Ratio Combinations European Medical Journal
Insulin Glp 1 Agonist Combinations Ppt Download
2

Efficacy And Safety Of Short And Long Acting Glucagon Like Peptide 1 Receptor Agonists On A Background Of Basal Insulin In Type 2 Diabetes A Meta Analysis Diabetes Care

Pdf Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial
Achieving Postprandial Glucose Control With Lixisenatide Improves Glycemic Control In Patients With Type 2 Diabetes On Basal Insulin A Post Hoc Analysis Of Pooled Data Springerlink

Beyond Basal Insulin How Glucagon Like Peptide 1 Receptor Agonists Fit In The Spectrum Of Therapeutic Options Consultant360




